How to Determine the Most Acidic Proton
The ratio for lower row elements is more even so the extra positive charges in the nucleus the extra protons in the nucleus means that were able to stabilize a negative charge more readily. Hydrogens directly attached to very electronegative atoms such as oxygen sulphur and the halogens carry a.
Solved Identify The Most Acidic Proton In This Molecule Chegg Com
The most acidic proton is the one that when removed forms the most stable conjugate base and that typically means where the negative charge can be delocalised.

. Identifying Acidic Protons 1. Therefore the first step is to look for all OH and NH bonds. The conjugate acid of a is a positively charged oxygen which as an approximate pKa -2.
When you have pi electrons delocalized around a single ring there is the 4n2 rule where the most stable configuration of pi electrons in a ring has 4n2 delocalized electrons. Y is an alkyl proton para to a carbonyl. The most acidic functional group usually is holding the most acidic H in the entire molecule.
1 Use the table of common acids that we learned in class and is in your book Table 31 inside front cover of the. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Hydrogens attached to a positively charged nitrogen oxygen or sulfur are acidic.
Also Im not entirely sure what effect the. Scan a molecule for known acidic functional groups. In the carboxylic acid the negative charge is distributed between two oxygens by resonance.
Z is an amine proton. A conjugate acid is formed when a proton is added to a base and a conjugate base is formed when a proton is removed from an acid. The high electronegativity of.
Cyclopentadiene and cycloheptatrienewhich is more acidic. Alkane alcohol carboxylic acid protonated. Its conjugate base is the weakest base here and.
Acids have a ph below 7. If instead you have. Whereas in the aminodicarbonyl the negative charge is interchanging.
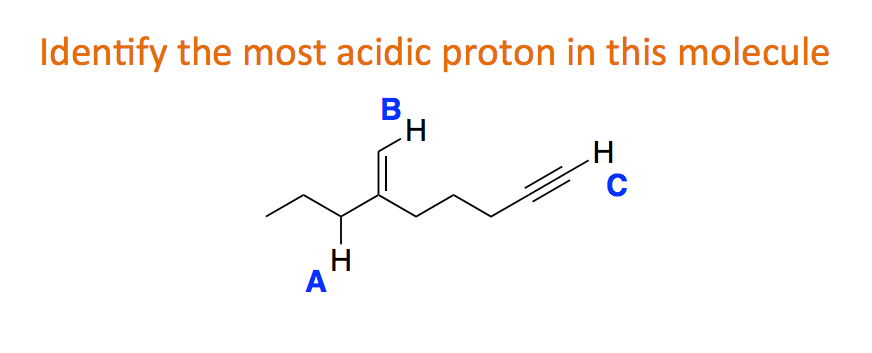
More is involved here than just resonance structure. Lone pairs on a neutral oxygen such as a and c are more stable than a lone pair on a negatively charged atom like b. This means the most acidic proton in this molecule is the on the terminal alkyne sp C-H.
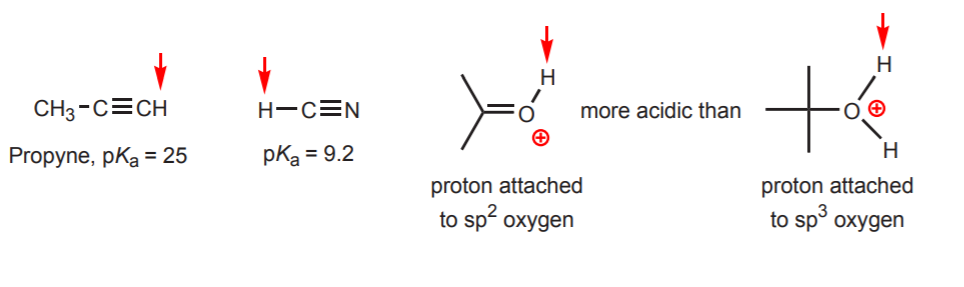
2 Identify which functional group the proton is a part of like. The hydrocarbons are generally considered very weak acids but among them the alkynes with a pKa 25 are quite acidic. Ascorbic acid also known as Vitamin C has a pK a of 41.
Therefore p should be the most acidic. A general guideline to determine if an oxide is acidic basic or amphoteric is to use the periodic table. There are three general methods to estimate the acidity of a proton.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. This makes the conjugate base more stable which means its proton is more acidic. For example you may need to determine which one of two double bondcontaining rings is more acidic such as the molecules shown here.
A planar conjugated ring with the proper 4n2 number of pi electrons is aromatic and will be strongly stabilized. 1 Use the table of common acids that we learned in class and is in your book Table 31 inside front cover of the. How to determine the most acidic proton in a given structure using ARIO.
36 picking the most acidic hydrogen in a molecule 37 How to find the most acidic H in a molecule 38 Ch 2 OHV Identifying the most acidic proton in a molecule. Acidic protons are usually bound to O or N. 2 Identify which functional group t.
Scan and rank sounds simple but it conceals several difficulties that are elaborated below. There are three basic methods of trying to estimate the acidity of a proton. Sp3 hybridized carbons are less acidic than sp2 which is less acidic than sp because the greater the s character of the bond the more stable is the negative charge of the carbanion.
X is an alkyl proton adjacent to a carbonyl. As evidenced by the pK a. The lone pair on an amine nitrogen by contrast is not part of a delocalized p system and is very ready to form a bond with any acidic proton that might be nearby.
Oxygen is more electronegative than nitrogen so it can stabilize the negative charge better. The molecule is Vitamin C ascorbic acid and the most acidic proton is the lower left. So on the molecule I drew is the negative charge better delocalised on the oxygen on the left or on the one on the right.
You can delocalize much more including the CC double bond and the ester group if you deprotonate there. As with any acid-base reaction in which you need to compare acidity you should look to see which acid has the more stable conjugate base. Sp3 hybridized carbons are more acidic than sp2 if the corresponding carbanions negative charge is distributed in a pi-system such as the allylic proton D.
P is an amide proton. Therefore as we go down the periodic table we tend to get to more acidic compounds because the conjugate base is more stable. DO NOT FORGET TO SUBSCRIBEThis video illustrates how to determine the most acidic proton as well as how to determine which molecule is more acidic based on.
You can delocalize much more including the CC double bond and the ester group if you deprotonate there. Often it requires some careful thought to predict the most acidic proton on a molecule. Therefore the lone pairs a and c are less basic than the lone pairs on b.
Ch 2 Ohv Identifying The Most Acidic Proton In A Molecule Youtube
11 10 Identifying Acidic Protons Chemistry Libretexts

Comments
Post a Comment